Venn Brown sat down with Brent Barnes, CEO and Managing Director of LBT Innovations, to discuss the company’s pivot into environment monitoring in the pharmaceutical manufacturing space, the launch of their new instrument, the AstraZeneca purchase, market size, the outlook of the pipeline, and the path to being cash flow positive.
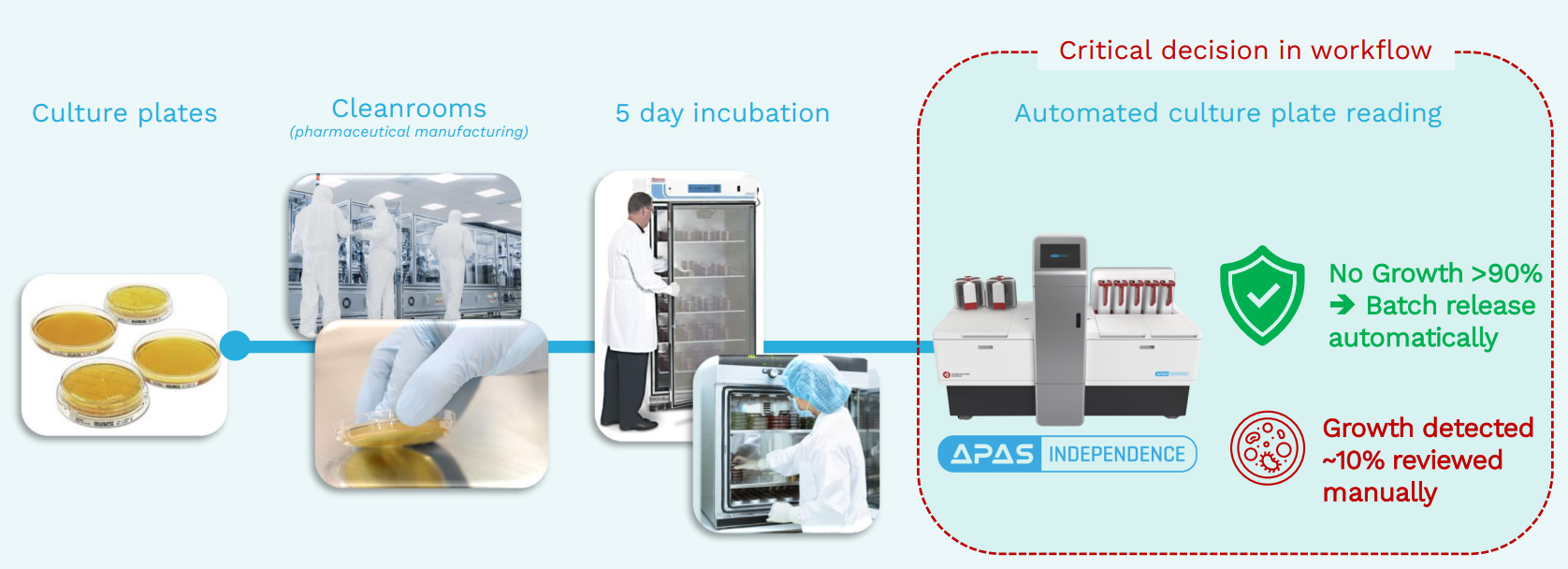
AI-powered environment monitoring: LBT Innovations is the maker of the Automated Plate Assessment System (APAS), an AI-powered machine that automates the examination of culture plates used to monitor the clean room environments of pharmaceutical manufacturing facilities. APAS replaces a manual process involving two-person teams who physically examine up to 1,000 plates a day for signs of bacterial growth.
Market size: LBT estimates a total addressable market worth around $2.8 billion. More than 14,000 laboratories and manufacturing facilities globally are mandated to use culture plates for environmental monitoring. The market is highly fragmented, with the 20 largest pharmaceutical companies and contract drug manufacturers operating approximately 600 facilities.
Competition: Two competitors in the market are currently installed in an estimated 150 facilities. LBT’s APAS technology offers greater scalability than existing solutions, with the APAS Independence able to process 1,600 plates a day compared to <150 plates a day from the competitor machines. APAS is either the same price or cheaper, takes up less space and aligns better with customers’ existing workflow.
Traction: In 2023, AstraZeneca gave LBT $1.2 million to accelerate APAS development. AstraZeneca has just purchased five devices worth approximately $5 million over five years. Two other top 10 pharma companies are currently evaluating the technology.
Outlook: LBT aims to be cash flow positive by FY26. Having only launched APAS in March, the company is just starting to build a footprint in the industry. The response has been positive, with AstraZeneca evangelising the system at various conferences and through published papers. The company reports a solid two-year pipeline of potential sales, including another 5 – 10 devices for AstraZeneca.
APAS processes 200 plates an hour with higher accuracy than manual reviews