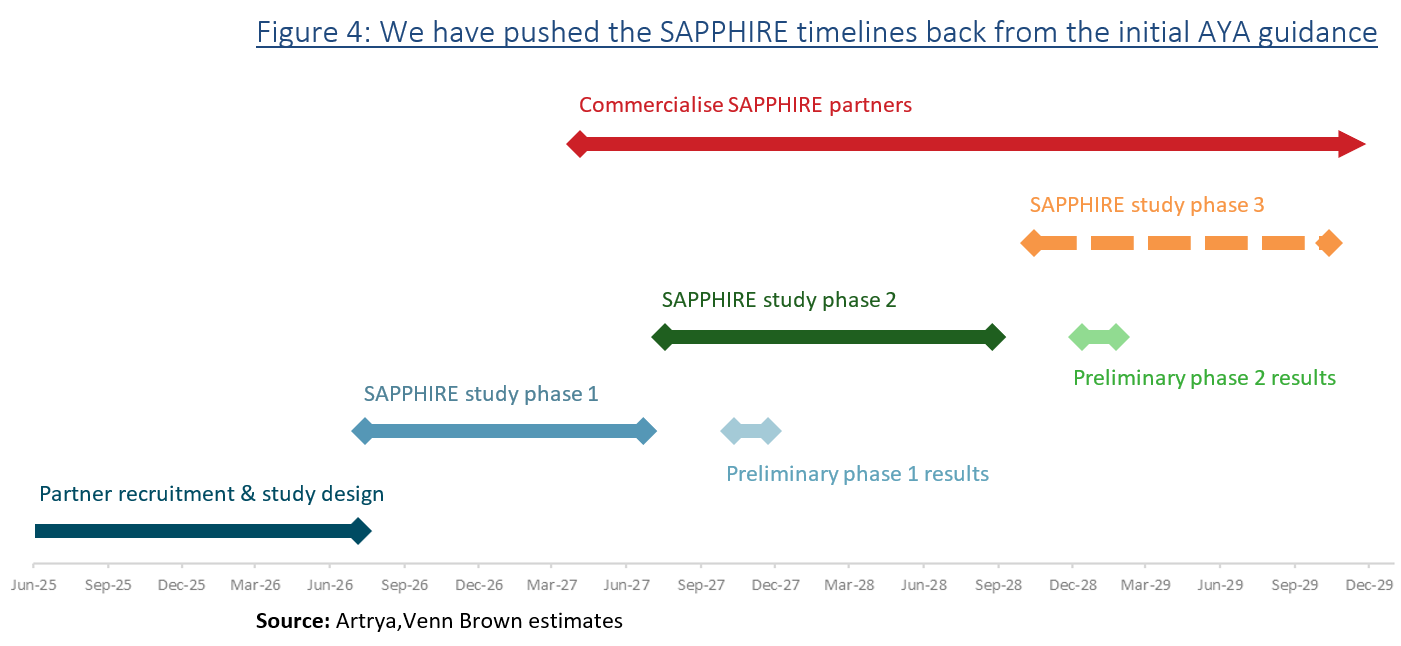
First announced in February 2025, the three-phase Salix-based Analysis of Plaque to identify Patients at HIgher Risk of Events (SAPPHIRE) study is a retrospective study examining whether AI-driven analysis is a superior predictor of heart attacks compared to current standard-of-care risk models.
The study involves processing CCTA scans of past or existing patients of partner hospital systems through Salix, and comparing the resulting risk assessment and diagnosis with those undertaken when the patient initially presented. There are material benefits for Artrya in running the study:
SAPPHIRE partners are not only large, but also prestigious. The study includes Mass General, one of the most prestigious hospital groups and medical research institutions in the world. Similarly, Piedmont Healthcare, Ascension and Huntsville are all regarded as leading cardiac research centres. Ascension is also the seventh-largest US healthcare system.
We suspect AYA is in discussions with at least one other top ten healthcare system. Management has reported several times that, combined, the study participants with which they’re in discussions complete around 400,000 CCTA scans a year, which could generate between $400 million and $500 million annually for Artrya. We asked Gemini to rank the ten largest healthcare systems in order of most to least likely to join SAPPHIRE. You can see its response in the full report.
In November 2025, Artrya announced Dr Ron Blankstein as the Principal Investigator of the SAPPHIRE study. Dr Blankstein’s involvement in the study adds enormous credibility to the study and to Salix.
Dr Blankstein is a leading cardiac researcher, a Professor of Medicine and Radiology at Harvard Medical School, the Associate Director of the Cardiovascular Imaging Program, Director of Cardiac Computed Tomography, and a Senior Preventive Cardiology Specialist at Mass General Brigham and Women’s Hospital.

SAPPHIRE is an expansion of an early AYA study completed in Australia that showed positive results. These earlier results provide confidence in the outcome of SAPPHIRE, which could lead to a change in the recommended diagnostic and risk assessment protocol of patients presenting with cardiac symptoms, which would include the use of a near-real-time, point-of-care AI-driven plaque qualifying and quantifying analysis, of which Salix is currently the only solution.
If the outcomes of the SAPPHIRE study match those of a previous study using Artrya's algorithm, the results could establish a new standard for diagnosing coronary artery disease and assessing patients presenting with cardiac distress.
For clinicians, it shifts the treatment paradigm from reactive (treating blockages) to preventative (treating high-risk plaque before a rupture). For healthcare systems, it validates the use of AI to automate complex scan readings, significantly improving throughput and diagnostic accuracy.
It should be noted that, even if Salix (and therefore AI systems) are shown to be meaningfully more effective than current diagnostic methods, it will still take several years for the recommended protocol to be developed and several years after that for it to be widely adopted.
Salix is currently the only AI-driven system providing near-real-time, point-of-care assessment, but competitors will emerge.