Artrya reported its quarterly results, which were broadly in line with expectations. Including the $30 million term deposit, AYA has $76.5 million in cash with a further $5.6 million expected in March from the R&D tax rebate.
The biggest news from the release is the delay of Salix Coronary Flow with AYA continuing its “calibration and refinement”. Management had originally expected to submit it to the FDA for clearance in the December quarter. This is now likely pushed to late 3Q26, with the company not expecting clearance until late June. This is a quarter later than we expected, but has no material impact on our forecasts.
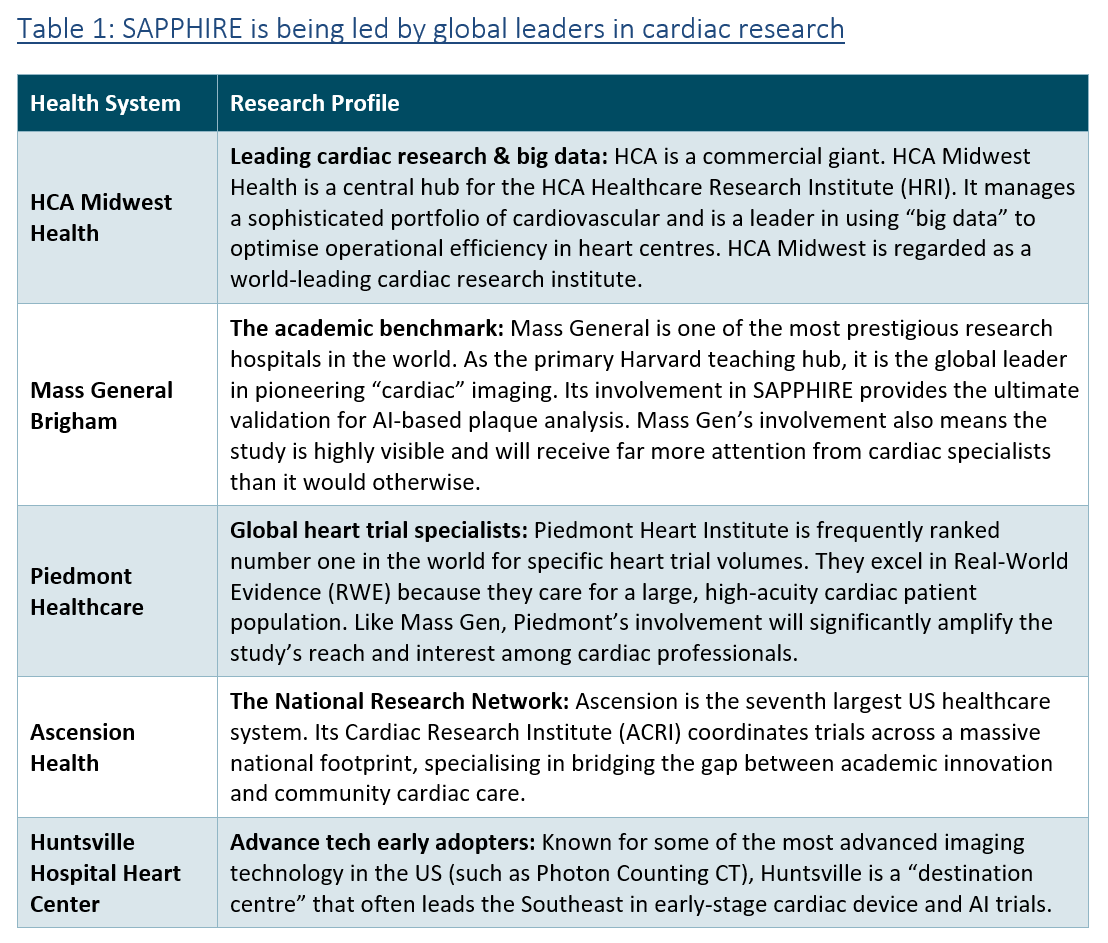
As discussed in several previous reports (www.vennbrown.com/artrya), it was a busy quarter, with highlights including the signing of five major US healthcare systems to the SAPPHIRE study, including yesterday’s announcement that HCA Midwest Health has joined the study. HCA Midwest is a global-leading cardiac research institution, and a division of HCA Healthcare, the largest healthcare system in the US (see ‘SAPPHIRE: HCA Midwest joins the party’)
AYA will host a results call on Monday 2nd February at 11am AEST / 8am AWST: https://artrya.zoom.us/webinar/register/WN_2It-WgGiTna4rHq6yzZZdA.
AYA continues to trade below our valuation. Future catalysts include: launch of the SAPPHIRE study, lodgement of the SCF FDA application, SCF FDA clearance, and reporting its first six months of US SCP revenue in 2H26.