We update our forecasts following yesterday’s announcementof the FDA’s clearance of Salix Coronary Plaque (reported here),the release of the group’s full-year results and today’s investor call.
FY25 results were broadly in line with expectations. Management is progressing integrations and commercial agreements with US partners; cash burn is expected to moderate in FY26, with FY27 still expectedto be broadly breakeven; Salix Coronary Flow remains on track for submission to the FDA by the end of the year; and design of the SAPPHIRE study continues and is expected to launch in early 2026.
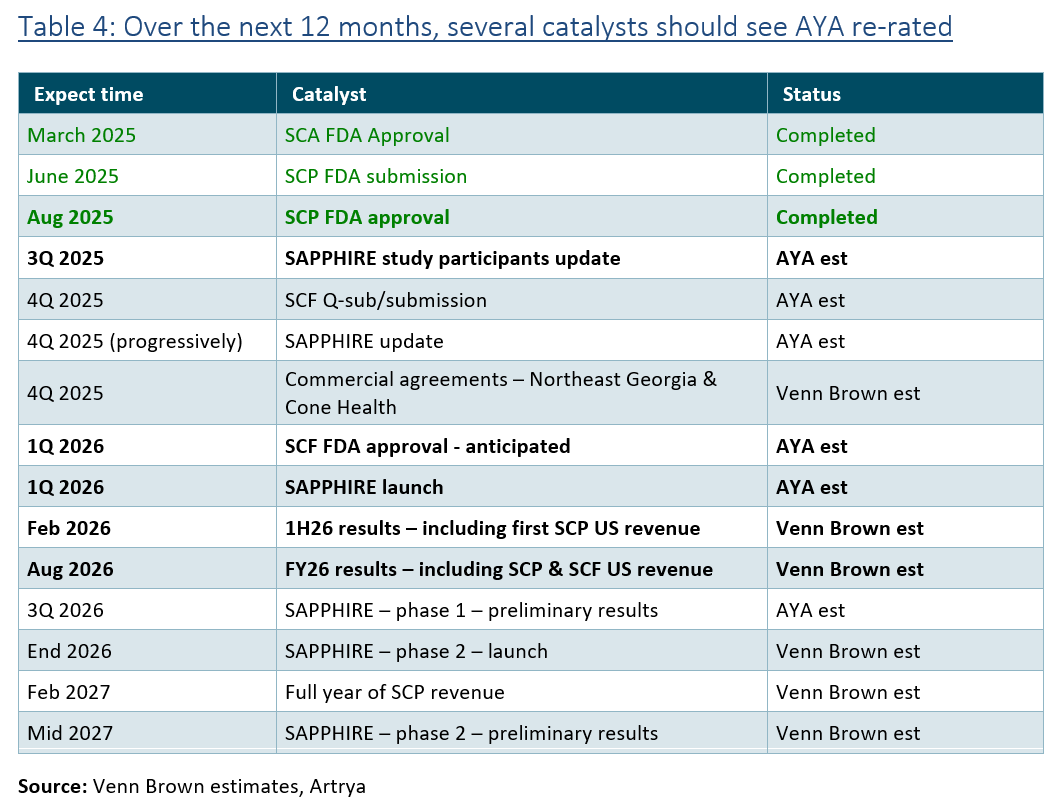
We’ve increased our valuation to $3.48 per share, based on adiscounted cash flow. The uplift is a result of increased expected revenue beyond FY28 based on positive industry feedback and Heartflow performance; slight cost reductions; and a reduction of the discount rate from 20% to 19% following the clearance of Salix Coronary Plaque.
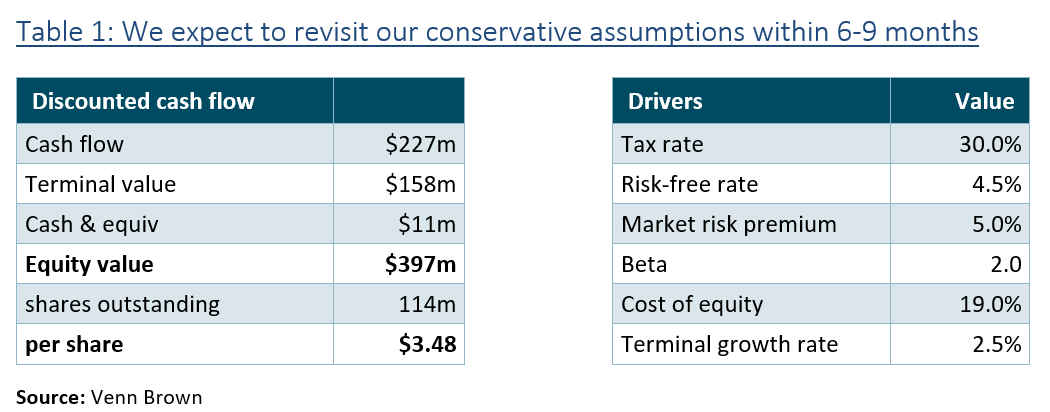
Management confirmed that they are still speaking with 6-8 health systems interested in participating in the SAPPHIRE study, which, combined, complete 400,000 CCTA scans a year. If the SAPPHIRE study progresses, we will revisit our valuation. A conservative valuation of AYA processing 400,000 scans a year is $35 per share. Assuming a four-year rollout and applying a 20% discount implies a current value of $14.60 per share.
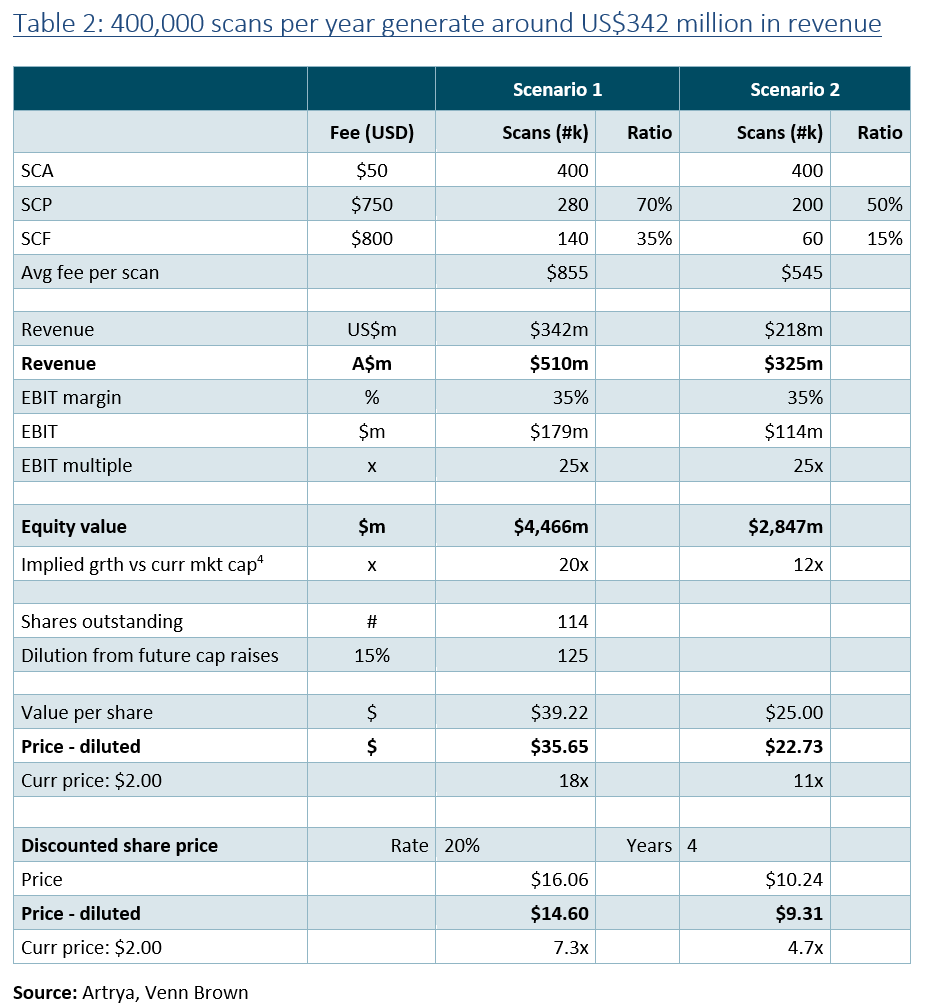
Having received FDA clearance for Salix Coronary Plaque the group’s has now cleared its largest and most significant risk hurdle. With the approval achieved, it now comes down to execution.
Looking ahead, the next twelve months will see Artrya achieve several other milestones that should serve as catalysts for the group’s underlying value to be better reflected in its share price, the key being:
As with any company, and especially small caps, the market will respond to performance, so depending on when AYA finalises its commercial agreements with its existing three US partners, and starts charge the full US$750 fee for SCP, we could start seeing meaningful revenue generation in the December quarter and reported in January 2026.